The macronutrient metabolism vitamins and minerals are:
Thiamin
Riboflavin
Niacin
Pantothenic Acid
Vitamin B6
Biotin
Vitamin B12
Vitamin C
Iodine
Manganese
Magnesium
All but three of these will be covered in this section. Vitamin B12 will be covered in the one-carbon metabolism chapter, vitamin C was covered in the antioxidant chapter, and magnesium is going to be covered in the electrolyte chapter. We’re left with iodine, manganese, and many of the B vitamins. We’ll cover the 2 minerals followed by the B vitamins with the order for the sections as follows:
10.1 Iodine
10.2 Manganese
10.3 Thiamin
10.4 Riboflavin
10.5 Niacin
10.6 Pantothenic Acid
10.7 Vitamin B6
10.8 Biotin
10.1 Iodine
Why is iodine first in this chapter? Not only is it the only non-B vitamin in this chapter, but there is also a connection between selenium (last antioxidant in the previous chapter) and iodine. Iodine’s only, yet critical, function is that it is required for thyroid hormone synthesis. The figure below shows that the thyroid gland is a butterfly-shaped organ found in the neck. The parathyroid glands are also found within the thyroid gland.

Iodine is found in foods primarily as iodide (I-), some bread dough has iodate (IO3-) added to help with gluten cross-linking2. This used to be more commonly used in the past than it is now. Like selenium, iodide concentrations of the soil vary greatly, causing food concentrations to greatly fluctuate. Sea water is high in iodine, thus foods of marine origin, such as seaweed and seafood, are good dietary sources of iodine. Dairy products also tend to be good sources of iodide because it is added to cattle feeds. Cattle receive iodine-containing medications, and iodide-containing sanitizing solutions are used in dairy facilities3,4.
For most Americans, we consume ample iodine through the consumption of iodized salt. Consumption of 1/2 teaspoon of iodized salt meets the RDA for iodine. There is a global logo for iodized salt. However, I must admit that I don’t recall ever seeing it myself.
The other link below is to a page that contains a scorecard map that depicts access to iodized salt worldwide. It also contains a Youtube video that displays the reduction in iodine deficiency over the last 2 decades.
| Web Link |
Salt is iodized with either potassium iodide (KI) or potassium iodate (KIO3). The positives of each are:
Potassium iodide
+ Less expensive
+ Higher iodine content (76% vs. 59% for KIO3)
+ More soluble
Potassium Iodate
+ More stable
The U.S. uses potassium iodide, but the form, and amount, used varies from country-to-country. Most Americans’ salt intake comes from processed foods, many of which are made with non-iodized salt. Iodine is well absorbed (~90%). Some dietary compounds interfere with thyroid hormone production or utilization. These compounds are known as goitrogens5. However, it is not believed that goitrogens are of clinical importance unless there is a coexisting iodine deficiency5.
Some examples of foods that contain goitrogens are3,4,6:
Cassava



Millet


Cruciferous Vegetables (broccoli, cabbage, Brussels sprouts)
Onions
Garlic
Soybeans
Peanuts
Subsections:
10.11 Thyroid Hormone
10.12 Iodine Deficiency & Toxicity
References & Links
- http://en.wikipedia.org/wiki/File:Illu_thyroid_parathyroid.jpg
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.
- Whitney E, Rolfes SR. (2008) Understanding nutrition. Belmont, CA: Thomson Wadsworth.
- Anonymous. (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academies Press.
- http://en.wikipedia.org/wiki/File:Casava.jpg
- http://en.wikipedia.org/wiki/File:Manihot_esculenta_dsc07325.jpg
- http://en.wikipedia.org/wiki/File:PeeledCassava.jpg
- https://en.wikipedia.org/wiki/Millet#/media/File:Grain_millet,_early_grain_fill,_Tifton,_7-3-02.jpg
- https://en.wikipedia.org/wiki/Staple_food#/media/File:Pearl_millet_after_combine_harvesting.jpg
Links
Global Iodized Salt Logo – https://en.wikipedia.org/wiki/File:Global_iodized_salt_logo.jpg
Global Iodine Scorecard – http://www.ign.org/scorecard.htm
10.12 Iodine Deficiency & Toxicity
There are two iodine deficiency disorders (IDD): goiter and cretinism. Goiter is a painless deficiency condition that results from the enlargement of the thyroid to help increase its ability to take up iodine. A couple of pictures of goiter are shown below.

A more serious consequence of iodine deficiency occurs during pregnancy to the fetus. Iodine deficiency during this time can lead to the cognitive impairment and stunted growth and known as cretinism. This condition is characterized by severe hypothyroidism, speech loss, and paralysis3,4. The following links show some examples of individuals with cretinism.
| Web Link |
The World Health Organization calls iodine deficiency “the world’s most prevalent, yet easily preventable, cause of brain damage5.” By saying it is easily preventable, they are referring to the ability of salt iodization to prevent brain development problems. The following New York Times article talks about how salt iodization may be the cheapest way to raise the world’s IQ.
| Web Link |
Iodine toxicity is rare, but like iodine deficiency, it can result in thyroid enlargement, and hypothyroidism or hyperthyroidism. Acute toxicity results in gastrointestinal irritation, abdominal pain, nausea, vomiting, and diarrhea6.
References & Links
- http://en.wikipedia.org/wiki/File:Kone_med_stor_struma.jpg
- http://en.wikipedia.org/wiki/File:Goitre.jpg
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
- http://www.who.int/nutrition/topics/idd/en/
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
Links
Cretinism – http://www.gsi.ir/Images/MedicalGeology/cretinism1.jpg
In Raising the World’s I.Q., the Secret’s in the Salt – http://www.nytimes.com/2006/12/16/health/16iodine.html?_r=1&pagewanted=all
10.2 Manganese
We know far less about manganese than many other minerals. Like many minerals it serves as a cofactor for a number of enzymes that are discussed in more detail below.
The enzyme superoxide dismutase uses manganese as a cofactor to convert superoxide to hydrogen peroxide as shown below.

In addition, both the enzymes involved in the gluconeogenesis oxaloacetate workaround use manganese as cofactors as shown below.

One enzyme in the urea cycle uses manganese as a cofactor.

Enzymes critical to the production of proteoglycans, which are essential components of cartilage and bone, use manganese as a cofactor5.

Absorption of manganese is not well understood but is believed to be pretty low (<5%). The main route (90%) of excretion is via bile excreted in feces5. Deficiency and toxicities of manganese are extremely rare. The deficiency is so rare in humans that there isn’t much information available on the symptoms of the condition. Symptoms in those who were deliberately made deficient include vomiting, dermatitis, changes in hair color, & skeletal defects5. Toxicity symptoms include neurological disorders similar to schizophrenia and Parkinson’s disease. In Chilean miners exposed to Mn-containing dust the toxicity was named Manganese Madness7,8.
References & Links
- http://en.wikipedia.org/wiki/Image:CellRespiration.svg
- http://commons.wikimedia.org/wiki/File:Liver.svg
- http://upload.wikimedia.org/wikipedia/commons/b/b0/Kidney_section.jpg
- http://en.wikipedia.org/wiki/File:Urea.png
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- http://en.wikipedia.org/wiki/File:Illu_synovial_joint.jpg
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
10.3 Thiamin
Thiamin (Vitamin B1) structurally consists of 2 rings that are bridged together as shown below.

Since it was one of the original vitamins, (remember vitamine), it was originally named thiamine. The -e has since been dropped in its spelling. Thiamin is sensitive to heat, so prolonged heating causes the cleavage of thiamin between the 2 rings destroying its activity2.
Like most of the B vitamins, thiamin’s primary function is as a cofactor for enzymes. It is not thiamin alone that serves as a cofactor but instead thiamin diphosphate (thiamin + 2 phosphates), which is more commonly referred to as thiamin pyrophosphate (TPP). The structure of thiamin pyrophosphate is shown below.

In plants, thiamin is found in its free form, but in animals it is mostly thiamin pyrophosphate. These phosphates must be cleaved before thiamin is taken up into the enterocyte4.
Thiamin uptake and absorption is believed to be an efficient process that is passive when thiamin intake is high and active when thiamin intakes are low4. There are two thiamin transporters (THTR), THTR1 and THTR2, that are involved in thiamin uptake and absorption. THTR1 is found on the brush border and basolateral membrane, while THTR2 is only found on the brush border membrane as shown below5.

Like most water-soluble vitamins there is little storage of thiamin.
Subsections:
10.31 Thiamin Functions
10.32 Thiamin Deficiency & Toxicity
References & Links
- http://en.wikipedia.org/wiki/File:Thiamin.svg
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.
- http://en.wikipedia.org/wiki/File:Thiamine_diphosphate.png
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Said H, Mohammed Z. (2006) Intestinal absorption of water-soluble vitamins: An update. Curr Opin Gastroenterol 22(2): 140-146.
10.31 Thiamin Functions
There are three functions of thiamin1:
- Cofactor for decarboxylation reactions (TPP)
- Cofactor for the synthesis of pentoses (5-carbon sugars) and NADPH (TPP)
- Membrane and nerve conduction (Not as a cofactor)
Decarboxylation Reactions
A decarboxylation reaction is one that results in the loss of carbon dioxide (CO2) from the molecule as shown below.

The transition reaction and one reaction in the citric acid cycle are decarboxylation reactions that use TPP as a cofactor. The figure below shows the transition reaction and citric acid cycle.

As shown below the conversion of pyruvate to acetyl CoA in the transition reaction is a decarboxylation reaction that requires TPP as a cofactor. CO2 (circled) is produced as a result of this reaction.

A similar TPP decarboxylation reaction occurs in the citric acid cycle converting alpha-ketoglutarate to succinyl-CoA. CO2 (circled) is given off as a result of this reaction.

TPP also functions as a cofactor for the decarboxylation of valine, leucine, and isoleucine (branched-chain amino acids)1.
Synthesis of Pentoses and NADPH
TPP is a cofactor for the enzyme transketolase. Transketolase is a key enzyme in the pentose phosphate (aka hexose monophosphate shunt) pathway. This pathway is important for converting 6-carbon sugars into 5-carbon sugars (pentose) that are needed for synthesis of DNA, RNA, and NADPH. In addition, pentoses such as fructose are converted to forms that can be used for glycolysis and gluconeogenesis4. Transketolase catalyzes multiple reactions in the pathway as shown below.

Membrane and Nerve Conduction
In addition to its cofactor roles, thiamin, in the form of thiamin triphosphate (TTP, 3 phosphates), is believed to contribute in some unresolved way to nervous system function1.
References & Links
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- https://commons.wikimedia.org/wiki/File:Decarboxylation_reaction.png
- http://en.wikipedia.org/wiki/File:Citric_acid_cycle_with_aconitate_2.svg
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
- https://commons.wikimedia.org/wiki/File:PPP_(en).svg
10.32 Thiamin Deficiency & Toxicity
Thiamin deficiency is rare in developed countries, but still occurs in poorer countries where white (aka polished) rice is a staple food. During the polishing process, thiamin, and many other nutrients, are removed. Some people also have a mutation in THTR1 that causes them to become thiamin deficient1. Thiamin deficiency is known as beriberi, which, when translated, means “I can’t, I can’t.” The symptoms of beriberi are illustrated in the link below.
| Web Link |
There are two major forms of beriberi: dry and wet. Dry beriberi affects the nervous system, with symptoms such as loss of muscle function, numbness, and/or tingling. Wet beriberi affects the cardiovascular system resulting in pitting edema, along with enlargement of the heart1. A picture of a person with beriberi is shown below.

Another group that is at risk for thiamin deficiency is alcoholics. There are three reasons why alcoholics are prone to becoming deficient3:
- Alcohol displaces foods that are better sources of thiamin
- Liver damage decreases TPP formation
- Increased thiamin excretion
The thiamin deficiency found in alcoholics is known as Wernicke-Korsakoff Syndrome. Symptoms of this condition include paralysis or involuntary eye movement, impaired muscle coordination, memory loss and confusion3. The following video shows some of the symptoms of this condition.
| Web Link |
Thiamin toxicity has never been reported as a result of oral intake. Thus, there is little worry about thiamin toxicity4.
References & Links
- http://www.nlm.nih.gov/medlineplus/ency/article/000339.htm
- http://en.wikipedia.org/wiki/File:Beriberi_USNLM.jpg
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
Links
Beriberi – http://www.moondragon.org/health/graphics/beriberi1.jpg
Wernicke-Korsakoff Syndrome – http://www.youtube.com/watch?v=wDcyBXJAZNM
10.4 Riboflavin
A student once asked this question:
“I started taking the Mega Man Sport Multi-vitamin from GNC and about an hour or two after consumption, with a meal, my pee is bright, practically neon yellow. What does that mean?”
Since this question is leading off the riboflavin section, you have probably surmised that riboflavin is somehow involved. Indeed, flavin means yellow in Latin, and riboflavin is bright yellow as shown below.

Riboflavin is also a water-soluble B vitamin, so the student was excreting large amounts of riboflavin in his urine, leading it to become “bright, practically neon yellow.” The structure of riboflavin is shown below.

Riboflavin is important for the production of two cofactors: flavin adenine dinucleotide (FAD) & flavin mononucleotide (FMN).
FAD has been introduced before, but structurally you can see where riboflavin is within the compound below.

The 2 circled nitrogens are the sites that accept hydrogen to become FADH2 as illustrated below.

The structure of FMN as shown below, is similar to FAD, except that it only contains one phosphate group (versus 2) and doesn’t have the ring structures off the phosphate groups that are found in FAD.

Riboflavin is photosensitive, meaning that it can be destroyed by light. This was a problem in the old days when the milkman delivered milk in clear glass bottles. These have now been replaced by cartons or opaque plastic containers to help protect the riboflavin content of the milk.

Riboflavin in foods is free, protein-bound, or in FAD or FMN. Only free riboflavin is absorbed so it must be cleaved, or converted before absorption6. Riboflavin is highly absorbed through an unresolved process, though it is believed a carrier is involved7. As you would guess from the description above, riboflavin is primarily excreted in the urine.
Subsections:
10.41 Riboflavin Functions
10.42 Riboflavin Deficiency & Toxicity
References & Links
- http://en.wikipedia.org/wiki/File:Riboflavin_solution.jpg
- http://en.wikipedia.org/wiki/File:Riboflavin.svg
- http://en.wikipedia.org/wiki/File:Flavin_adenine_dinucleotide.png
- http://en.wikipedia.org/wiki/File:FAD_FADH2_equlibrium.png
- http://en.wikipedia.org/wiki/File:Flavin_mononucleotide.png
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Said H, Mohammed Z. (2006) Intestinal absorption of water-soluble vitamins: An update. Curr Opin Gastroenterol 22(2): 140-146.
10.41 Riboflavin Functions
Riboflavin is required for the production of FAD and FMN. Below are some of the functions of FAD and FMN1:
- Citric Acid Cycle
- Electron Transport Chain
- Fatty Acid Oxidation
- Niacin Synthesis
- Vitamin B6 Activation
- Neurotransmitter Catabolism
- Antioxidant Enzymes
- Citric Acid Cycle – FAD is reduced to FADH2 in the citric acid cycle when succinate is converted to fumarate by succinic dehydrogenase as circled below.

- Electron Transport Chain – Under aerobic conditions, the electron transport chain is where the FADH2 is used to produce ATP. Complex I of the electron transport chain includes an FMN molecule. The electron transport chain is shown below.

- Fatty Acid oxidation – During fatty acid oxidation FAD is converted to FADH2 as shown below.

- Niacin synthesis – As you will hear more about in the niacin section, niacin can be synthesized from tryptophan as shown below. An intermediate in this synthesis is kynurenine, and one of the multiple steps between kynurenine to niacin requires FAD.

- Vitamin B6 Activation – The enzyme that creates the active form of vitamin B6 (pyridoxal phosphate) requires FMN.

- Neurotransmitter Catabolism – The enzyme monoamine oxidase (MAO) requires FAD. This enzyme shown below is important in the catabolism of neurotransmitters such as dopamine and serotonin.


- Antioxidant Enzymes – The antioxidant enzymes glutathione reductase and thioredoxin reductase both require FAD as a cofactor. Thioredoxin reductase is a selenoenzyme. The function of glutathione reductase is shown in the following link. Glutathione reductase can reduce glutathione that can then be used by the selenoenzyme glutathione peroxidase to convert hydrogen peroxide to water.
| Web Link |
In addition to the functions listed above, FAD is also used in folate activation, choline catabolism, and purine metabolism1.
References & Links
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
2. http://en.wikipedia.org/wiki/File:Citric_acid_cycle_with_aconitate_2.svg
3. http://en.wikipedia.org/wiki/File:Mitochondrial_electron_transport_chain%E2%80%94Etc4.svg
4. https://commons.wikimedia.org/wiki/File:Nicotinic_acid_biosynthesis2.png
5. http://en.wikipedia.org/wiki/File:Pyridoxine_structure.svg
6. https://en.wikipedia.org/wiki/Pyridoxal_phosphate#/media/File:Pyridoxal-phosphate.svg
7. http://en.wikipedia.org/wiki/File:Dopamine_degradation.svg
8. http://en.wikipedia.org/wiki/File:Serotonin_biosynthesis.svgLinks
The Glutathione Oxidation Reduction (Redox) Cycle – http://lpi.oregonstate.edu/infocenter/minerals/selenium/gsh.html
10.42 Riboflavin Deficiency & Toxicity
Ariboflavinosis, riboflavin deficiency, is a rare condition that often occurs with other nutrient deficiencies. The symptoms of this condition are shown in the figure below.

The most notable symptoms include angular stomatitis (aka angular cheilitis, like cheilosis), which is a lesion that forms at the corners of the mouth as shown below.

Glossitis is the inflammation of the tongue, which can be accompanied by redness or inflammation of the oral cavity. Dermatitis is also frequently a symptom3,4.
There has been no toxicity of riboflavin reported.
References & Links
- http://www.fao.org/docrep/W0073e/p206.gif
- http://en.wikipedia.org/wiki/File:Angular_Cheilitis.JPG
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.
10.5 Niacin
There are two forms of niacin: nicotinic acid and nicotinamide (aka niacinamide), that have a carboxylic acid group or amide group, respectively. The structure of nicotinic acid and nicotinamide are shown below.


Niacin is important for the production of two cofactors: nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP+). The structure of NAD is shown below; you can clearly see the nicotinamide at the top right of the molecule.

NAD is reduced to form NADH, as shown below.

The structure of NADP+ is exactly the same as NAD, except it has an extra phosphate group off the bottom of the structure, as shown below.

Like NAD, NADP+ can be reduced to NADPH.
Niacin is unique in that it can be synthesized from the amino acid tryptophan as shown below. An intermediate in this synthesis is kynurenine. Many reactions occur between this compound and niacin, and riboflavin and vitamin B6 are required for two of these reactions.
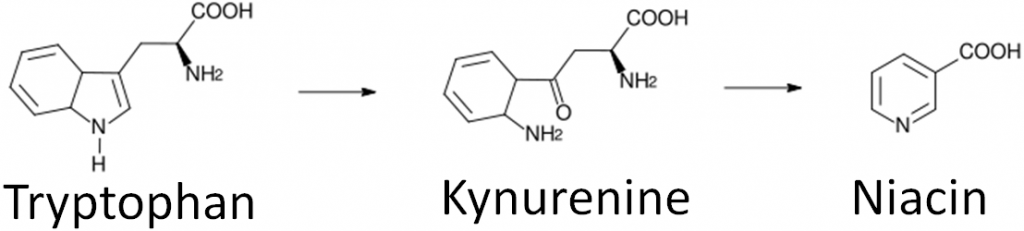
To account for niacin synthesis from tryptophan, niacin equivalents (NE) were created by the DRI committee to account for the amount of niacin in foods as well as their tryptophan content. It takes approximately 60 mg of tryptophan to make 1 mg of niacin. Thus, the conversions to niacin equivalents are:
1 mg Niacin = 1 NE
60 mg Tryptophan = 1 NE
The tryptophan levels of most foods is not known, but a good estimate is that tryptophan is 1% of amino acids in protein7. Thus, lets take peanut butter, smooth style, with salt as an example8.
The peanut butter contains 13.403 mg of niacin and 25.09 g of protein8.
Step 1: Calculate the amount of tryptophan:
25.09 g X 0.01 (the numerical value of 1%) = 0.2509g of tryptophan
Step 2: Convert Grams to Milligrams
0.2509 g X 1000 mg/g = 250.9 mg of tryptophan
Step 3: Calculate NE from tryptophan
250.9 mg of tryptophan/(60 mg of tryptophan/1 NE) = 4.182 NE
Step 4: Add NEs together
13.403 NE (from niacin) + 4.182 (from tryptophan) = 17.585 NE
Most niacin we consume is in the form of nicotinamide and nicotinic acid9, and in general is well absorbed using an unresolved carrier10. However, in corn, wheat, and certain other cereal products, niacin bioavailability is low. In these foods, some niacin (~70% in corn) is tightly bound, making it unavailable for absorption. Treating the grains with a base frees the niacin and allows it to be absorbed. After absorption nicotinamide is the primary circulating form7,9.
Subsections:
10.51 Niacin Functions
10.52 Niacin Deficiency & Toxicity
References & Links
- http://en.wikipedia.org/wiki/File:Niacinstr.png
- http://en.wikipedia.org/wiki/File:Nicotinamide_structure.svg
- http://en.wikipedia.org/wiki/File:NAD%2B_phys.svg
- http://en.wikipedia.org/wiki/File:NAD_oxidation_reduction.svg
- http://en.wikipedia.org/wiki/File:NADP%2B_phys.svg
- https://commons.wikimedia.org/wiki/File:Nicotinic_acid_biosynthesis2.png
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.
- http://www.nal.usda.gov/fnic/foodcomp/search/
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Said H, Mohammed Z. (2006) Intestinal absorption of water-soluble vitamins: An update. Curr Opin Gastroenterol 22(2): 140-146.
10.51 Niacin Functions
Approximately 200 enzymes require NAD or NADP+. We will go through some selected functions of NAD and NADP+. The following figures and legends show and describe the functions of NAD and NADP+.





HMG CoA reductase, the rate-limiting enzyme in cholesterol synthesis, uses NADPH. NADPH is also used by the antioxidant enzyme glutathione reductase as shown in the link below6.
| Web Link |
References & Links
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
- http://en.wikipedia.org/wiki/File:Citric_acid_cycle_with_aconitate_2.svg
- http://en.wikipedia.org/wiki/File:Ethanol_flat_structure.png
- https://en.wikipedia.org/wiki/Acetaldehyde#/media/File:Acetaldehyde-2D-flat.svg
- http://en.wikipedia.org/wiki/Mitochondrion
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
Links
The Glutathione Oxidation Reduction (Redox) Cycle – http://lpi.oregonstate.edu/infocenter/minerals/selenium/gsh.html
10.52 Niacin Deficiency & Toxicity
Pellagra is a niacin deficiency. This is no longer a common deficiency in developed countries, but was in the U.S. in the early 1900s. This was because corn was a staple crop, meaning it was what people primarily consumed. The bioavailability of niacin from corn is poor unless treated with a base to release the bound niacin. The symptoms of pellagra are the 3 D’s:
Dementia
Dermatitis
Diarrhea
Some refer to 4 D’s in which the 4th D is death if the condition is not managed. The following pictures show the symptoms of pellagra.


The link below shows another picture of a person suffering from dermatitis as a result of pellagra.
| Web Link |
Dietary niacin toxicity is rare. However, nicotinic acid (not nicotinamide) can improve people’s lipid profiles when consumed at levels far above the RDA. For instance the RDA & upper limit (UL) is 14 or 16 (women & men) and 35 mg (both), respectively. Many people are taking 1-2 grams (up to 6 g/day) to get the benefits in their plasma lipid profiles as shown in the table below4,5.
Table 10.521 Effects of nicotinic acid (>1.5 g/day) on plasma lipid profile3
| Measure | Change |
| VLDL | ↓ 25-40% |
| LDL | ↓ 6-22% |
| HDL | ↑ 18-35% |
| Total Cholesterol | ↓ 21-44% |
| Triglycerides | ↓ 21-44% |
It should be pointed out that there are special supplements for this purpose that include a slower release nicotinic acid that helps prevent the toxicity symptoms (nicotinamide is not toxic). A slow (aka long or extended) release form of niacin for people with atherosclerosis is Niaspan®. The link below is to Niaspan’s site.
| Web Link |
A study found that Niaspan plus a statin was no better than a statin alone in preventing heart attacks, despite improvements in HDL and triglyceride levels. This result challenged the understanding of the importance of HDL and triglyceride levels to heart attack risk. The link below explains this study’s results.
| Web Link |
The most well known of the toxicity symptoms is “niacin flush”, which is a dilation of capillaries accompanied by tingling that can become painful. This symptom is noted to occur at lower levels than the other toxicity symptoms6. Other symptoms include:
Gastrointestinal Distress
Liver Damage
A nicotinic acid receptor GPR109A, which is present in adipocytes and immune cells, is believed to mediate niacin flush, but the beneficial effects on lipid profiles do not appear to be mediated by it7,8. It is not clear at this time the mechanism of action for the improvements in lipid profiles7. Thus, nicotinic acid supplementation can improve lipid profile and lead to niacin flush, while nicotinamide supplementation does not result in either outcome.
References & Links
- http://www.fao.org/docrep/w0073e/p184.gif
- http://en.wikipedia.org/wiki/File:Pellagra_NIH.jpg
- Gille A, Bodor E, Ahmed K, Offermanns S. (2008) Nicotinic acid: Pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol 48: 79-106.
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- Whitney E, Rolfes SR. (2008) Understanding nutrition. Belmont, CA: Thomson Wadsworth.
- Ginsberg HN, Reyes-Soffer G.. (2013) Niacin: A long history, but a questionable future. Curr Opin Lipidol. 24: 475-479.
- Liu D, Wang X, Kong L, Chen Z. (2015) Nicotinic acid regulates glucose and lipid metabolism through lipid-independent pathways. Curr Pharm Biotechnol. 16: 3-10.
Links
Pellagra – http://www.pathguy.com/lectures/mcgill_pellagra.jpg
Niaspan® – http://www.niaspan.com/
Niaspan® Health Care Professional’s Site – http://www.niaspanpro.com/
Niacin Drugs Don’t Reduce Heart Attack Risk – http://www.nytimes.com/2011/05/27/health/policy/27heart.html
10.6 Pantothenic Acid
Pantothenic acid has two roles in the body:
- It is part of coenzyme A (CoA)
- It is part of acyl carrier protein
- Coenzyme A
The structure of pantothenic acid is shown alone below and circled within coenzyme A.


The functions of CoA are shown and described below3.



- Acyl Carrier Protein
Acyl carrier protein, is also important in fatty acid synthesis3.
Most pantothenic acid in food is found as CoA, which is cleaved prior to absorption. It is then taken up into the enterocyte through the sodium-dependent multivitamin transporter (SMVT) as shown below. Approximately 50% of pantothenic acid is absorbed; it is excreted primarily in urine3.

Deficiency of pantothenic acid is very rare. Pantothenic acid supplementation did relieve the symptoms (burning feet and numbness of toes) of “burning feet syndrome” in prisoners in World War II6. It is believed pantothenic acid deficiency was the cause of this syndrome. Other symptoms noted are vomiting, fatigue, weakness, restlessness, and irritability3. No toxicity has been reported.
References & Links
- http://en.wikipedia.org/wiki/File:Pantothenic_acid_structure.svg
- http://en.wikipedia.org/wiki/File:Coenzym_A.svg
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
- https://en.wikipedia.org/wiki/File:Animal_mitochondrion_diagram_en_%28edit%29.svg
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
10.7 Vitamin B6
Vitamin B6 is composed of three compounds: pyridoxine, pyridoxal, and pyridoxamine. Pyridoxine contains a methylhydroxyl group (-CH3OH), pyridoxal an aldehyde (-CHO), and pyridoxamine an aminomethyl group (-CH3NH2), as shown below.



All three forms can be activated by being phosphorylated. The phosphorylated forms can be interconverted to the active, or the cofactor form of vitamin B6, pyridoxal phosphate (PLP). This active form has a phosphate group added in place of a hydroxyl group. The enzyme that catalyzes this reaction requires FMN (riboflavin cofactor), as shown below.

In animal products, vitamin B6 is found in its cofactor forms, PLP and pyridoxamine phosphate (PMP). The latter cofactor is less common than PLP. In plants, vitamin B6 is primarily found as pyridoxine, with up to 75% being pyridoxine glucoside, which is believed to be the plant storage form6. Pyridoxine glucoside has a glucose added to pyridoxine as shown below.

Vitamin B6 is well absorbed from foods (~75%) through passive diffusion. PLP and PMP are dephosphorylated before uptake into the enterocyte. Some of the pyridoxamine glucoside is cleaved to form free pyridoxine, but some pyridoxine glucoside is absorbed intact. Pyridoxine glucoside absorption is lower (~50%) than pyridoxine alone. The primary circulating forms of vitamin B6 are pyridoxal and PLP. Vitamin B6 is primarily excreted in the urine, and like many other B vitamins, vitamin B6 is destroyed during cooking or heating6.
Subsections:
10.71 Vitamin B6 Functions
10.72 Vitamin B6 Deficiency & Toxicity
References & Links
- http://en.wikipedia.org/wiki/File:Pyridoxine_structure.svg
- http://en.wikipedia.org/wiki/File:Pyridoxal.png
- http://en.wikipedia.org/wiki/File:Pyridoxamine.png
- https://en.wikipedia.org/wiki/Pyridoxal_phosphate#/media/File:Pyridoxal-phosphate.svg
- http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=440188&loc=ec_rcs
- Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. (2006) Modern nutrition in health and disease. Baltimore, MD: Lippincott Williams & Wilkins.
10.71 Vitamin B6 Functions
PLP is a cofactor for over 100 different enzymes, most are involved in amino acid metabolism. In fact, without PLP, all amino acids would be essential because we would not be able to synthesize nonessential amino acids. Below are some of the functions of PLP and PMP1:



PLP is required for decarboxylase enzymes that are involved in the synthesis of the neurotransmitters GABA, serotonin, histamine, and dopamine. As an example, DOPA decarboxylase uses PLP to convert L-DOPA to dopamine as shown below1.

PLP is also required by gamma-aminolevulinic acid (ALA) synthetase that is involved in heme synthesis, as shown below. Heme will be discussed in more detail in the iron section.

PLP is also used in one of the multiple reactions that occurs between kynurenine and niacin in its synthesis from tryptophan.

In addition, PLP is also involved in:
Carnitine Synthesis
1-Carbon Metabolism
References & Links
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- http://en.wikipedia.org/wiki/File:Transaminierung.svg
- http://en.wikipedia.org/wiki/File:DesaminierungCtoU.png
- http://en.wikipedia.org/wiki/File:Catecholamines_biosynthesis.svg
- http://en.wikipedia.org/wiki/File:Heme_synthesis.png
- https://commons.wikimedia.org/wiki/File:Nicotinic_acid_biosynthesis2.png
10.72 Vitamin B6 Deficiency & Toxicity
Vitamin B6 deficiency is rare, but symptoms include:
Skin or scalp ailments (seborrheic dermatitis)
Microcytic hypochromic anemia (small cells, low color)
Convulsions
Depression
Confusion
Given what we know about the functions of vitamin B6 most of these symptoms make sense.
The microcytic hypochromic anemia is a result of decreased heme synthesis. The neurological symptoms are due to the decreased production of neurotransmitters1.
Vitamin B6, unlike many of the B vitamins, can produce toxicity. High doses of vitamin B6, taken for an extended period of time, can lead to neurological damage2. There are some potential uses of vitamin B6 supplementation that are important to be done with consultation with a physician.
One of the conditions that people take vitamin B6 for is carpal tunnel syndrome. The following video does a nice job of explaining and showing how this condition occurs.
| Web Link |
While the evidence is not conclusive, it appears that vitamin B6 supplementation may be beneficial to those suffering from carpal tunnel syndrome and may be tried alone, or in combination with other complementary treatments, before surgery is undertaken3,4.
Morning sickness that occurs early in pregnancy is another condition where vitamin B6 supplementation is utilized. The evidence again is not clear on whether it is beneficial5,6, but The American College of Obstetricians and Gynecologists suggests that vitamin B6 may be tried first to treat nausea and vomiting during pregnancy7. In 2013, the FDA approved doxylamine-pyridoxine (Diclegis) for use in pregnancy8. It is not known exactly what causes morning sickness, but it is believed that lower circulating vitamin B6 levels are associated with increased morning sickness severity9.
The last condition that vitamin B6 is commonly supplemented for is premenstrual syndrome (PMS). A systematic literature review found that it is inconclusive whether vitamin B6 supplementation is beneficial in managing PMS10.
References & Links
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
- Ryan-Harshman M, Aldoori W. (2007) Carpal tunnel syndrome and vitamin B6. Canadian Family Physician 53(7): 1161-1162.
- Aufiero E, Stitik T, Foye P, Chen B. (2004) Pyridoxine hydrochloride treatment of carpal tunnel syndrome: A review. Nutr Rev 62(3): 96-104.
- Koren G, Maltepe C. (2006) Preventing recurrence of severe morning sickness. Canadian Family Physician 52(12): 1545-1546.
- Tan P, Yow C, Omar S. (2009) A placebo-controlled trial of oral pyridoxine in hyperemesis gravidarum. Gynecol Obstet Invest 67(3): 151-157.
- http://www.acog.org/Patients/FAQs/Morning-Sickness-Nausea-and-Vomiting-of-Pregnancy
- Slaughter SR, Hearns-Stokes R, van der Vlugt T, Joffe HV. (2014) FDA approval of doxylamine-pyridoxine therapy for use in pregnancy. N Engl J Med. 370: 1081-1083.
- Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Tambunan V, Bardosono S. (2012) Vitamin B6 supplementation in pregnant women with nausea and vomiting. 116: 206-210.
- Whelan A, Jurgens T, Naylor H. (2009) Herbs, vitamins and minerals in the treatment of premenstrual syndrome: A systematic review. The Canadian Journal of Clinical Pharmacology 16(3): e407-e429.
Video
Carpal Tunnel Syndrome – http://www.youtube.com/watch?v=rewDQgqU5Hg
10.8 Biotin
The 2 primary dietary forms of biotin are free biotin and biocytin (aka biotinyllysine)1. The structure of biotin is shown below.

Biocytin is biotin bound to lysine as seen in its structure below.

Free biotin is believed to be highly absorbed. Before uptake, biocytin is acted on by the enzyme biotinidase, forming free biotin and lysine. Free biotin is then taken up into the enterocyte through the sodium-dependent multivitamin transporter (SMVT), as shown below1,4,5.

Most biotin is excreted in the urine.
Subsections:
10.81 Biotin Functions
10.82 Epigenetics
10.83 Biotin Deficiency & Toxicity
References & Links
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- http://en.wikipedia.org/wiki/File:Biotin_structure.svg
- http://commons.wikimedia.org/wiki/File:Biocytin.svg
- Said H, Mohammed Z. (2006) Intestinal absorption of water-soluble vitamins: An update. Curr Opin Gastroenterol 22(2): 140-146.
- Zempleni J, Wijeratne SSK, Hassan Y. (2009) Biotin. Biofactors 35(1): 36-46.
10.81 Biotin Functions
Biotin is an important cofactor for carboxylase enzymes. As the name sounds, these enzymes add carboxylic acid groups (-COOH) to whatever compound they act on. In fatty acid synthesis, biotin is required by the enzyme that forms malonyl CoA from acetyl-CoA, as shown below1.

Another biotin-requiring carboxylase is one that converts pyruvate to oxaloacetate in gluconeogenesis as shown below1.

In addition to these two functions, biotin is also important for histone biotinylation and the breakdown of isoleucine, leucine, methionine, and threonine1.
Histone biotinylation is an epigenetic modification that is described in the next section.
Biotin is an effective treatment for brittle nail syndrome, but it has not been shown to improve healthy nails4. There is little evidence to suggest that biotin improves healthy hair as well5.
References & Links
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- http://en.wikipedia.org/wiki/File:Animal_mitochondrion_diagram_en_%28edit%29.svg
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
- Scheinfeld N, Dahdah MJ, Scher R. (2007) Vitamins and minerals: their role in nail health and disease. 6(8): 782-787.
- Famenini S, Goh C. (2014) Evidence for supplemental treatments in androgenetic alopecia. 13(7): 809-812.
10.82 Epigenetics
What is epigenetics? Epigenetics means “above the genome.” The nucleotide sequence of the human genome is known, and there is surprisingly little difference between individuals. However, the 2 main epigenetic modifications play a major role in determining what genes are expressed:
DNA methylation
Histone modification
These epigenetic modifications are illustrated in the following link.
| Web Link
Epigenetic modifications (inactive link as of 05/18/2021) |
DNA methylation is the addition of a methyl group to a DNA base, which decreases gene transcription. Conversely, demethylation increases gene transcription.
DNA does not exist simply as long strands of double helix, instead it is packaged and shaped so that it can fit in the nucleus of our cells. The first part of this packaging is that DNA is wrapped around proteins called histones as shown below.

Histone modification occurs when there are additions or subtractions to the histones themselves. The most common is acetylation (addition of an acetyl group) or deacetylation of histones. The structure of acetyl is shown below.

Histone acetylation causes the DNA structure to open up so that transcription can occur. Histone deacetylation causes the DNA to become more tightly packed, preventing transcription from occurring.
Together, these modifications to DNA and histones are known as the epigenetic code. The following two videos do a good job explaining epigenetics and tying together its two different methods of modification:
| Web Links |
How does this relate to biotin? Histones can be biotinylated, or have biotin added to the histone. However, it should be noted that histone biotinylation is rare (<0.001% of human histones H3 and H4), so it is questionable how much impact this action has3.
References & Links
- http://en.wikipedia.org/wiki/File:Nucleosome_structure.png
2. http://en.wikipedia.org/wiki/File:Acetyl.svg - Kuroshi T, Rios-Avila L, Pestinger V, Wijeratne SSK, Zempleni J. (2011) Biotinylation is a natural, albeit rare, modification of human histones. Mol Genet Metab, 104, 537-545.
Links
Epigenetic modifications – http://www.nature.com/nature/journal/v441/n7090/images/441143a-i2.0.jpg
Videos
A Tale of Two Mice – https://www.youtube.com/watch?v=OOiCu5kzGxg
Histone Modifications – http://www.youtube.com/watch?v=eYrQ0EhVCYA
10.83 Biotin Deficiency & Toxicity
Biotin deficiency is very rare. Symptoms of biotin deficiency include1:
Skin rash
Hair loss
Neurological Impairments
There are a couple of ways that a person could develop a deficiency in biotin. First, a very small number of people are born with a mutation in biotinidase that results in them not being able to cleave biocytin for absorption1. Another way is through the consumption of raw eggs. Drinking raw eggs is not something that most people do. However, some people do it to imitate Sylvester Stallone’s movie character Rocky, who consumed them as part of his boxing training regimen. If you are not familiar with this movie the link below shows you how Rocky consumed his raw eggs.
| Web Link |
The potential problem with consuming raw eggs routinely is that raw egg whites contain a protein called avidin which binds biotin and prevents its absorption. However, it would take more than two dozen egg whites consumed daily over many months to cause a deficiency, making this an unlikely occurrence2. Cooking denatures avidin and prevents it from binding biotin, meaning that cooked eggs are not a concern.
No toxicity of biotin has been reported.
References & Links
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.
- Whitney E, Rolfes SR. (2008) Understanding nutrition. Belmont, CA: Thomson Wadsworth.
Videos
Rocky Raw Eggs – http://www.youtube.com/watch?v=NhkdLHSKo9s